Genmab’s DuoBody platform
I was interested in learning about the scientific core of Genmab’s business model. Looking for this, I found two key papers: the 2013 controlled Fab-arm exchange paper and the 2013 scale-up and manufacturing paper. The conceptual origin sits a bit earlier, in the IgG4 literature, where Fab-arm exchange shows up as a biological behavior that can be harnessed.
Genmab is an antibody company whose economics lean on partnered products and royalties, with a smaller slice coming from co-commercialized assets where they share development and sales. Their main value is in a set of engineering platforms that let them produce antibody formats partners already want, while staying close to standard IgG manufacturing workflows.
All of this had to start from some scientific underpinning, so let's get into the 2013 paper.
Bispecific antibodies
A bispecific antibody carries two different binding specificities, which is the whole point. You build bispecifics when two targets need to be engaged in a coordinated way, for example: physically bringing an immune cell to a tumor cell (CD3 on T cells plus a tumor antigen), blocking two signaling nodes at once to reduce pathway escape, or forcing a receptor complex into a particular geometry that changes the downstream biology. The problem is that the classical IgG architecture was built from symmetry (two identical heavy chains and two identical light chains), and that symmetry becomes a manufacturing liability as soon as you ask one molecule to carry two different binding arms.
To remind you (and myself) on the terminology: the Fab, fragment antigen-binding region, is the part that contacts the antigen and contains the variable regions that give specificity. The Fc, fragment crystallizable region, is the stem that recruits immune effector functions (the things an antibody can trigger after it binds its target) and controls half-life. Within the Fc, the CH3 domains sit at the heavy-chain interface and act as a key driver of the dimerization.
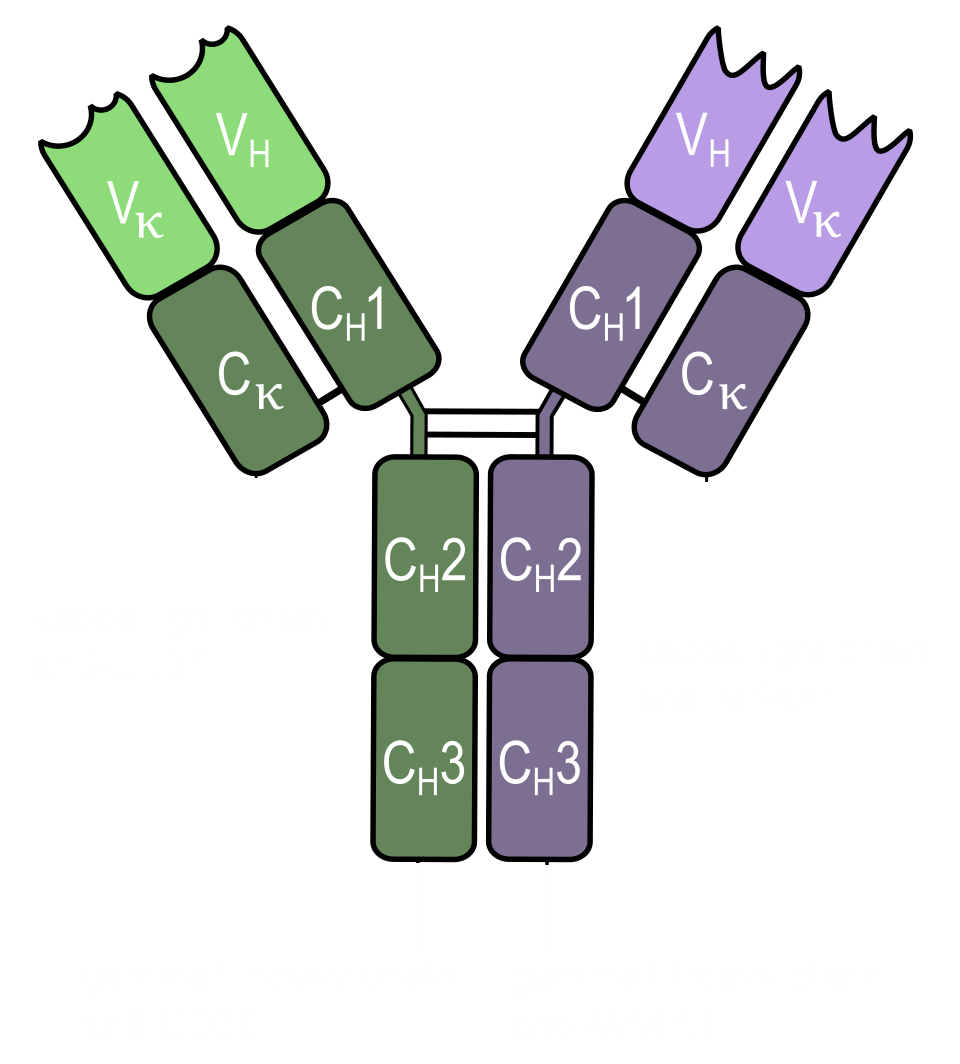
The problems that existed in producing bispecific antibodies is the following: when you express multiple heavy and light chains together, the cell assembles a mixture of pairings, so you spend a lot of effort effort separating desired product from close chemical siblings that look like antibodies because they are antibodies, simply arranged in the wrong combinations.
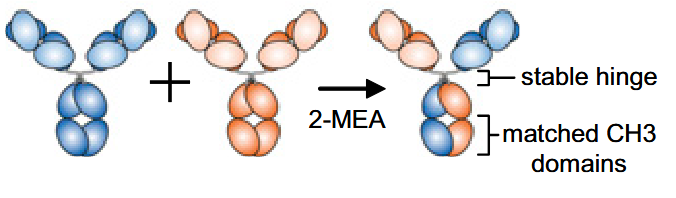
Genmab’s 2013 paper describes controlled Fab-arm exchange, the basis of their DuoBody platform, and uses a known IgG4 behavior to assemble stable IgG1 bispecifics under defined redox conditions. By doing this with IgG1 they were able to quickly convert this to medicine, as it is what manufacturers and pharmcokenticists are already familiar with.
Controlled Fab-arm exchange
The DuoBody method described in Labrijn et al., 2013 starts with an unusual premise: you avoid co-expression chaos by producing two perfectly ordinary monoclonals separately, each one homogeneous and manufacturable on its own using known methods, and you postpone bispecific assembly to a controlled in-vitro step. The engineering trick sits at the CH3–CH3 interface in the Fc, where Genmab uses a matched pair of point mutations on the two parental antibodies ( F405L on one parent and K409R on the other in the IgG1 examples used for scale-up). The heterodimeric Fc interface now becomes thermodynamically favored under the exchange conditions.
The idea now looks like something like this conceptually very simple plan:
- Produce the two parental IgG1 antibodies separately, using standard upstream and downstream platform approaches.
- Mix them at some specific ratio and add a reducing agent, commonly 2-MEA, so the disulfides break open and half-antibodies can reshuffle.
- Let the system re-equilibrate while the engineered CH3 interface biases the assembly toward the heterodimer.
- Remove the reductant so disulfides re-form and lock the structure into a stable IgG.
In order for such a plan to actually be usable in pharma, it needs to scale. In the Gramer et al., 2013 demonstration of facility fit, this exchange was pushed to manufacturing scale with parental antibodies produced at 1000 L, the exchange reaction run at tens of liters, and reported yields above 95% bispecific product at kilogram scale.
Why does this elegant approach work so well? I think it combines the strengths of biology (assembling the parents) and strengths of rational controlled chemistry (mutations and reduction) nearly perfectly. Using engineered CH3 pairing to bias the endpoint toward one major species is something I can really appreciate, it really is one simple trick to make your reaction work.
Genmab’s near future
With controlled Fab-arm exchange in hand, Genmab gained a repeatable way to make full-length IgG bispecifics whose developability profile stays close to the monoclonals they start from, which matters because it lets later work concentrate on target biology, dosing, and safety management instead of constant format reinvention. Epcoritamab, a subcutaneous CD3×CD20 IgG1 bispecific, is an example of a medication that came out of this technology.
DuoBody tech answers how to build a stable, manufacturable IgG that binds two things. Genmab now has their eyes set on bigger goals: their HexaBody aims to work on what the Fc does after binding. For this they use Fc engineering to amplify complement-driven activity through enhanced IgG hexamerization. But that's for another article.





